Abstract
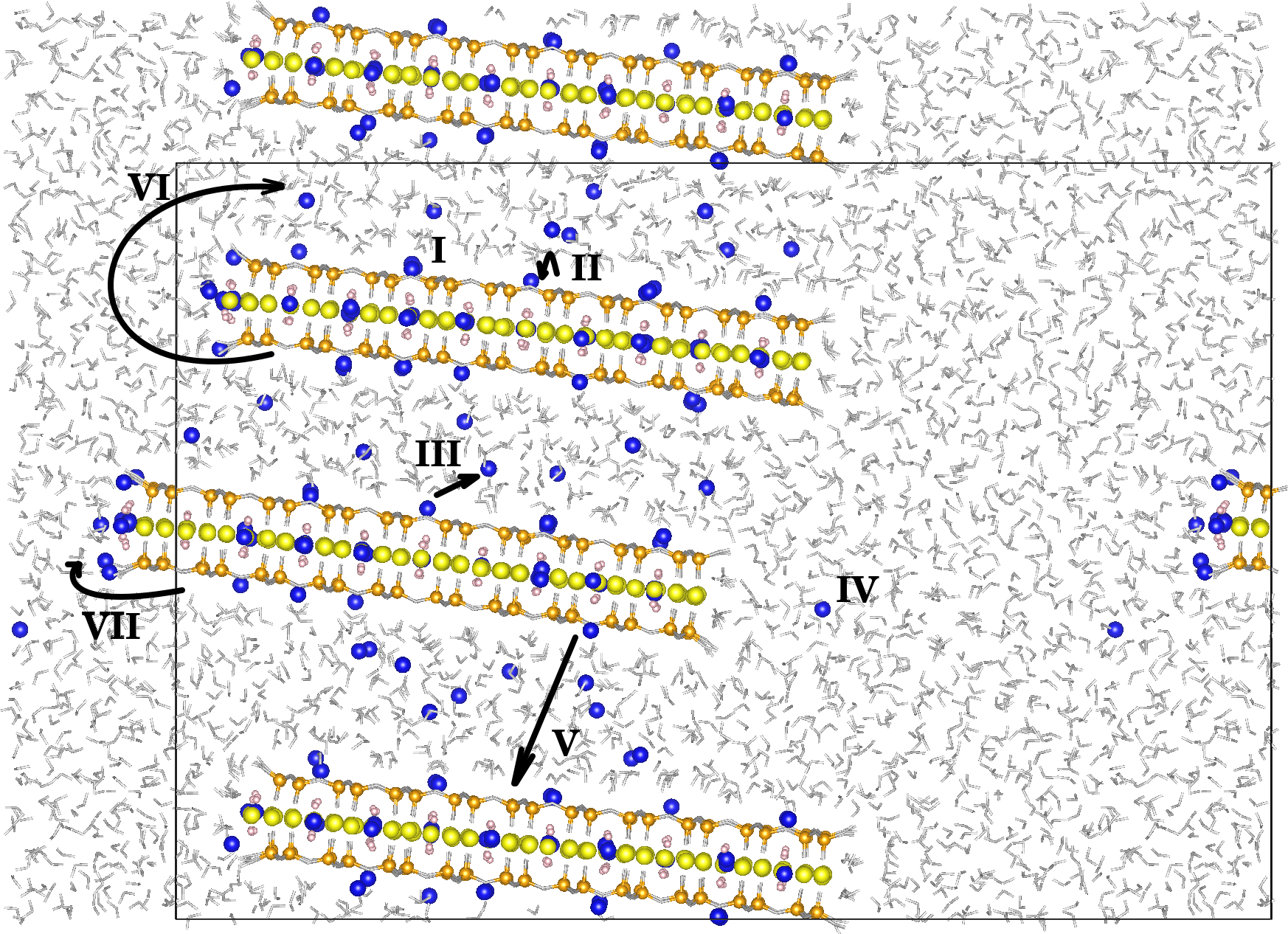
The charge of clays plays an important role in the mobility of compensating cations and in swelling processes. In this work we have developed a method to generate Lithium Fluorhectorite (Li-Fh) clay models with a charge of -1.2e and a non-homogeneous charge distribution. This charge is closer to the experimentally reported value. We used this approach to study their interaction with water using Molecular Dynamics (MD) simulations. The MD simulations showed that the Li+ diffusion coefficient increases by two orders of magnitude with decreasing clay charge. Population analyses and Li+ coordination indicate a greater interaction of the cations with water molecules with decreasing clay charge, leading to a deformation in the stacking of clay layers in the 010 model. These results highlight the important influence of clay charge on cation dynamics and structural behavior, providing insight into delamination and swelling mechanisms.
References
[1] F. Bergaya, B. K. G. Theng, y G. Lagaly, Handbook of Clay Science: Clay Mineral Control of Soil Organic Matter: A Conceptual Model Based on Physical Interactions (Elsevier, 2006).
[2] M. Yu, S. M. Tariq, y H. Yang, Clay Minerals 57, 51 (2022).
[3] S. Khan, S. Ajmal, T. Hussain, y M. U. Rahman, J. UQ. Univ. Appl. Sci. 9, 1 (2023).
[4] Y. Lan, Y. Liu, J. Li, D. Chen, G. He, y I. P. Parkin, Adv. Sci. 8, 2004036 (2021).
[5] A. C. D. Newman, Phil. Trans. R. Soc. Lond. A 311, 375 (1984).
[6] B. O. Otunola y O. O. Ololade, Environ. Tech. & Innov. 18, 100692 (2020).
[7] A. Ochirkhuyag y J. Temuujin, Minerals 14, 629 (2024).
[8] J. Dong, Z. Cheng, S. Tan, y Q. Zhu, Expert Opin. Drug Deliv. 18, 695 (2020).
[9] C. Aguzzi, P. Cerezo, C. Viseras, y C. Caramella, Appl. Clay Sci. 36, 22 (2007).
[10] M. M. Orta, J. Mart´ın, J. L. Santos, I. Aparicio, S. Medina-Carrasco, y E. Alonso, Appl. Clay Sci. 198, 105838 (2020).
[11] H. Kalo, M. W. M¨ oller, D. A. Kunz, y J. Breu, Nanoscale 4, 5633 (2012).
[12] A. Lam y G. Rojas-Lorenzo, Rev. Cubana Fis. 41, 10 (2024).
[13] R. P. Tenório, M. Engelsberg, J. O. Fossum, y G. J. da Silva, Langmuir 26, 9703 (2010).
[14] L. Michels, C. L. S. da Fonseca, Y. Méheust, M. A. S. Altoé, E. C. dos Santos, G. Grassi, R. Droppa Jr., K. D. Knudsen, L. P. Cavalcanti, K. W. B. Hunvik, J. O. Fossum, G. J. da Silva, y H. N. Bordallo, J. Phys. Chem. C 124, 24690 (2020).
[15] A. Lam y G. Rojas-Lorenzo, Rev. Cubana Fis. 39, 85 (2022).
[16] R. T. Cygan, J.-J. Liang, y A. G. Kalinichev, J. Phys. Chem. B 108, 1255 (2004).
[17] M. Pouvreau, J. A. Greathouse, R. T. Cygan, y A. G. Kalinichev, J. Phys. Chem. C 123, 11628 (2019).
[18] S. Koneshan, J. C. Rasaiah, R. M. Lynden-Bell, y S. H. Lee, J. Phys. Chem. B 102, 4193 (1998).
[19] I. Todorov,W. Smith, K. Trachenko, y M. Dove, J. Mater. Chem. 16, 1911 (2006).
[20] W. G. Hoover, Phys. Rev. A 31, 1695 (1985).
[21] S. Nosé, J. Chem. Phys. 81, 511 (1984).
[22] M. P. Allen y D. J. Tildesley, Computer Simulation of Liquids, 2nd ed. (Oxford University Press, Oxford, UK, 2017).
[23] P. E. Smith y B. M. Pettitt, Comput. Phys. Commun. 91, 339 (1995).
[24] F. Kraehenbuehl, H. F. Stoeckli, F. Brunner, G. Kahr, and M. Mueller-Vonmoos, Clay Minerals 1, 1 (1987).

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright (c) 2025 Cuban Physical Society & Faculty of Physics of the University of Havana